Explanation: Maxwell's Demon (Slides.1-3)
- Maxwell imagines one container divided into two parts, A and B. Both parts are filled with the same gas at equal temperatures and placed next to each other. Observing the molecules on both sides, an imaginary demon guards a trapdoor between the two parts. The demon opens the trapdoor when a faster-than-average molecule (●) in A flies towards the trapdoor, and let it go from A to B. But it does not do so when a slower-than-average molecule (●) comes. Conversely, when a slower-than-average molecule (●) from B flies towards the trapdoor, the demon will let it pass, but does not do so when a faster-than-average molecule (●) comes. The average speed of the molecules in B will have in creased while that in A they will have slowed down on average. Since temperature in an enclosure is related to the average kinetic energy of the particles contained in it, this will result in the creation of a temperature gradient between the two compartments.
- Thus we've redistributed the random kinetic energy of the molecules (heat) in such a way that energy can now be extracted from the system (e.g., to drive a gas turbine) violating the Second law of thermodynamics. The demon has managed to decrease the entropy the system.
Slide 1 Maxwell's demon lnitial state
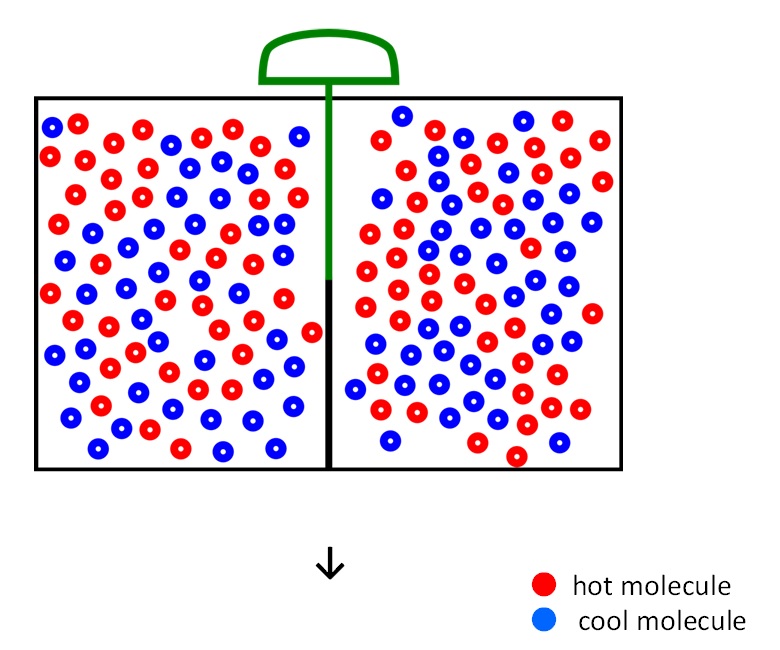
(1/3)
Slide 2: Sorting by the demon
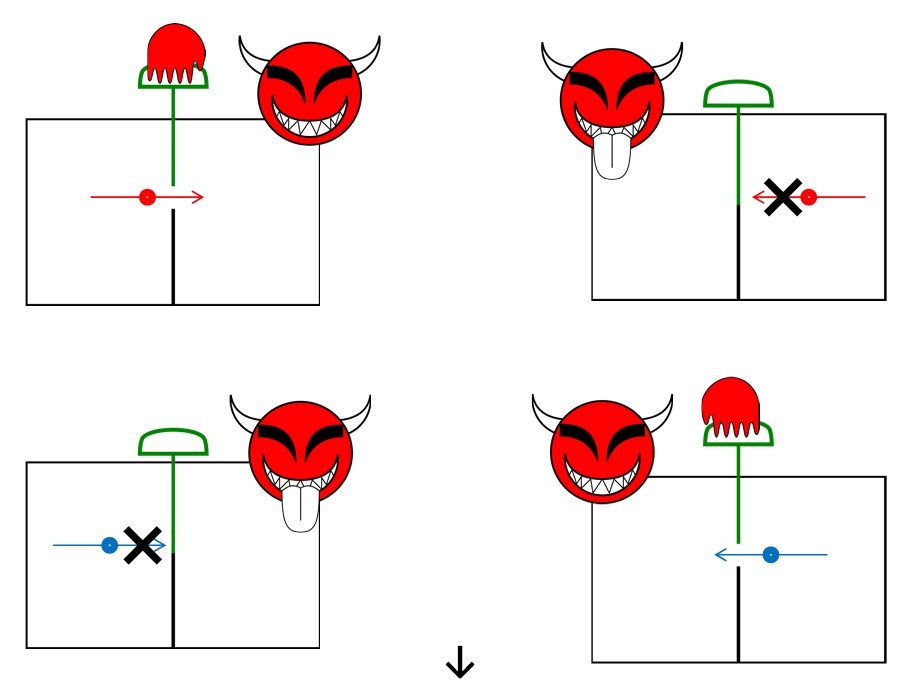
(2/3)
Fig. 3 Final state
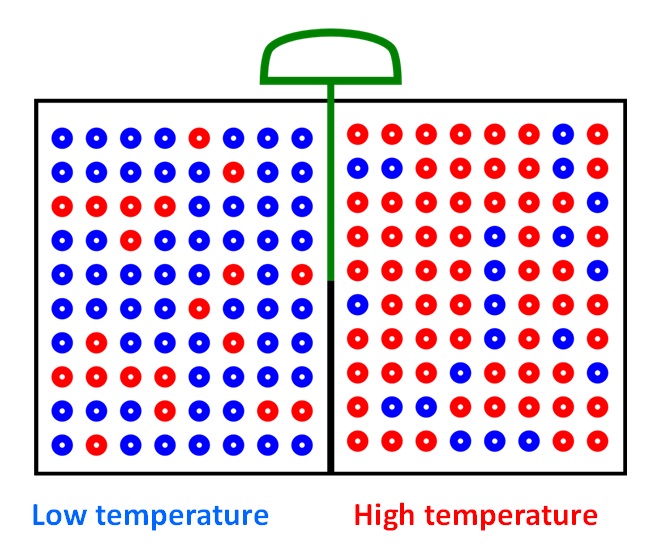
(3/3)
We will able to extract free energy from an isothermal system with the help of a demon